The cell and gene therapy CROs market is anticipated to grow at an annualized rate of 18% by 2035, claims Roots Analysis
Characterized by high prevalence rate of rare and genetic disorders, and rise in demand for effective novel ATMPs, the cell and gene therapy contract research industry is likely to witness evolutionary advancements, in the coming years.
Roots Analysis has announced the addition of Cell and Gene Therapy CRO Market , 2022-2035” report to its list of offerings.
In order to mitigate the challenges associated with the cell and gene therapies research and development, around 50-60% of the developers prefer to outsource their operations to contract research organizations (CROs), which claim to have the required expertise and experience to leverage their capabilities and yield cost savings opportunities. The aforementioned challenges are believed to be the key factors for driving the outsourcing of research operations to a cell and gene therapy CRO , which claim to be well-aware of the nuances of advanced therapy medicinal products (ATMP) design and development, as well as cell and gene therapy manufacturing process.
To request a sample copy / brochure of this report, please visit
https://www.rootsanalysis.com/....reports/230/request-
Key Market Insights
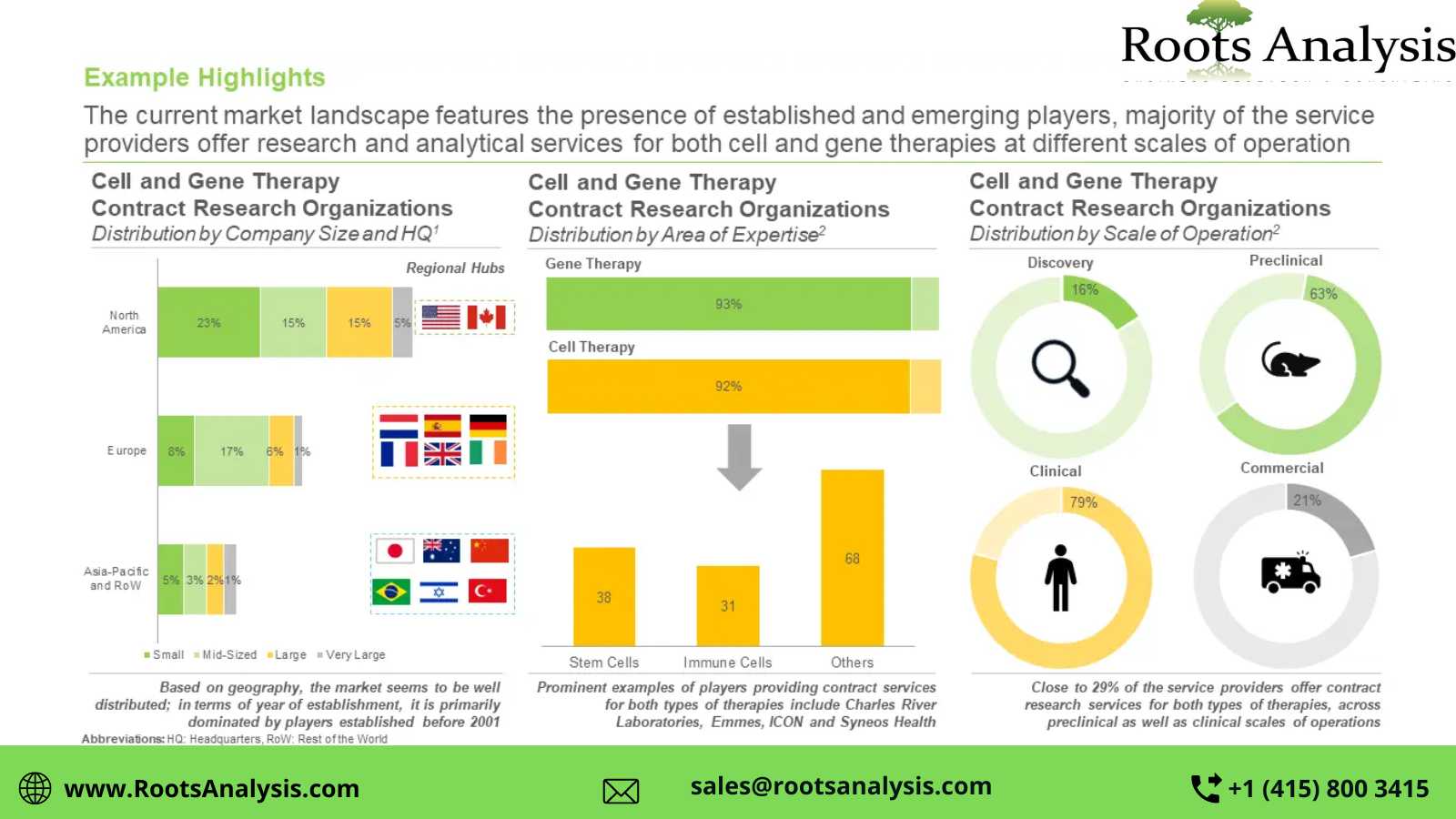
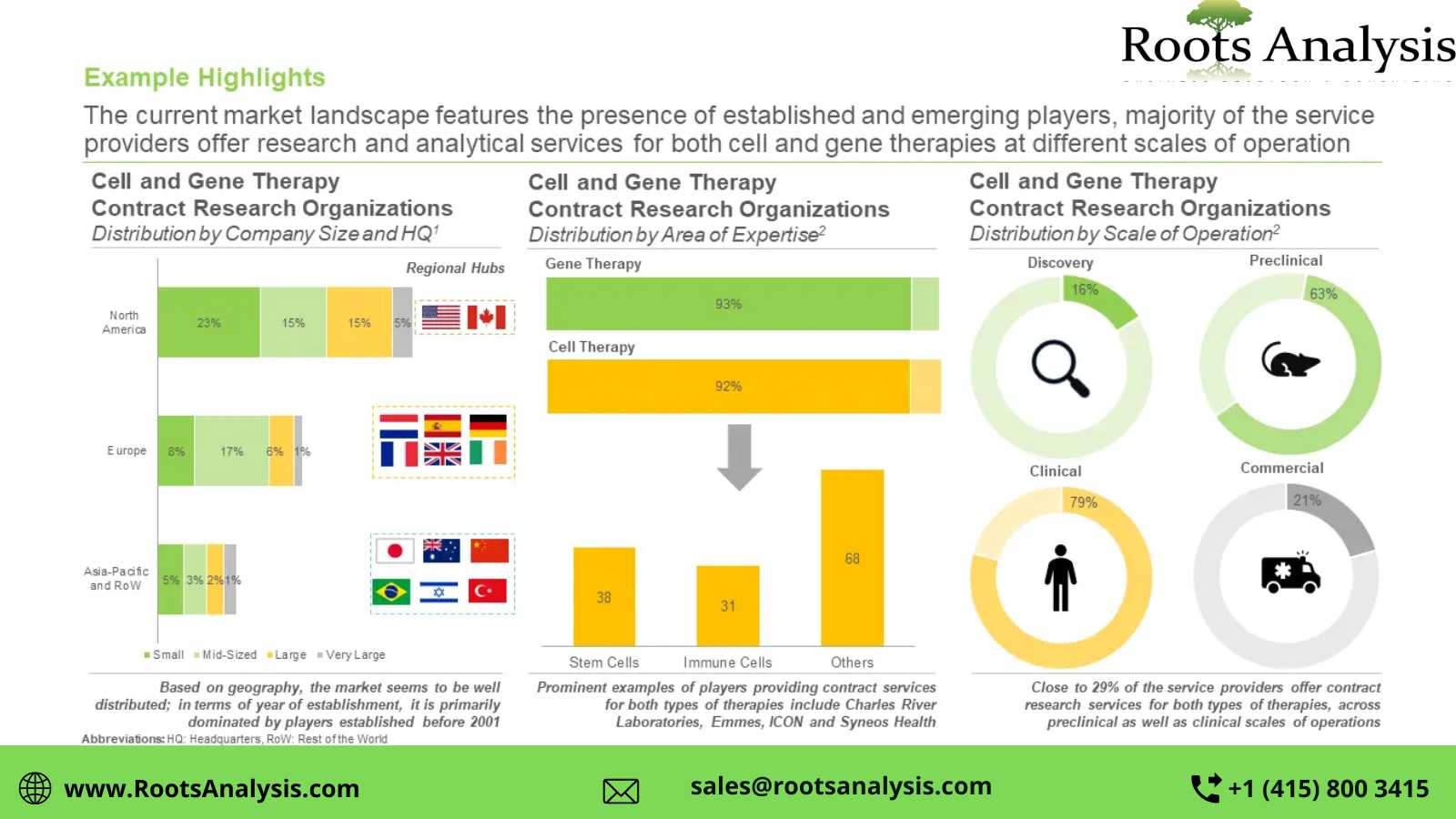
Presently, more than 105 players claim to offer cell and gene therapy contract research services
This segment of the industry is dominated by the presence of mid-sized (51-500 employees) and small (2-51 employees) players, which collectively represent more than 70% of the total CROs in this domain. In addition, about 20% of the firms were founded post 2010.
>40% of the players carry out contract research operations across preclinical and clinical scales
Nearly 21% of the total industry stakeholders have the required capabilities for cell and gene therapies at the commercial scale. In addition, 16% CROs are capable of providing contract research services for discovery purposes.
Partnership activity in this domain has increased at a CAGR of over 61%, during 2015- 2022
Acquisitions and mergers emerged as the most popular type of partnership model adopted by industry stakeholders (54%), followed by service agreements (20%). Further, most of the deals were inked by players based in North America (58%).
Over 32 mergers and acquisitions were established in this domain, during the period 2015-2022
It was observed that majority of the deals were intracontinental (72%), involving participants from different countries. Geographical consolidation and portfolio addition were observed to be the major key value drivers of M&A activity, followed by service portfolio and geographical expansion (28%, each).
More than 1,330 clinical trials evaluating cell and gene therapies have been registered worldwide
Of the total, close to 79% of the studies are active and recruiting patients, followed by those that have already been completed (7%). It is important to highlight that more than 20% of cell and gene therapy focused-clinical trials are currently in phase II.
North America and Europe are anticipated to capture over 75% of the market share, in 2035
In terms of scale of operation, the current market is driven by clinical operations (55%); this trend is unlikely to change in the foreseen future as well. Further, based on area of expertise, majority of the revenue share (61%) of the overall market in 2035 is likely to be driven by cell therapies.
For additional details, please visit
https://www.rootsanalysis.com/....reports/view_documen
You may also be interested in the following titles:
1. Gene Therapy Market: (5TH Edition) Industry Trends and Global Forecasts, 2022-2035
2. RNA Sequencing Services Market: Industry Trends and Global Forecasts, 2022-2035
About Roots Analysis
Roots Analysis is one of the fastest growing market research companies, sharing fresh and independent perspectives in the bio-pharmaceutical industry. The in-depth research, analysis and insights are driven by an experienced leadership team which has gained many years of significant experience in this sector.
Contact:
Ben Johnson
+1 (415) 800 3415
ben.johnson@rootsanalysis.com